Aldehydes and Ketones are molecules that have the functional group of C=O.
An aldehyde has a carbon bonded to a hydrogen and an oxygen whereas a ketone has an oxygen bonded to a carbon which is bonded to two other carbons.
The oxygen is more electronegative than the carbon making the oxygen δ- and the carbon δ+.
Aldehydes Name Formula methanal HCHO ethanal CH3CHO propanal CH3CH2CHO methylpropanal CH3CH(CH3)CHO 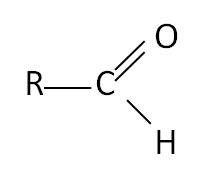 | Ketones Name Formula propanone CH3COCH3 butanone CH3CH2COCH3 pentan-2-one CH3CH2CH2COCH3 3-methylbutan-2-one CH3COCH(CH3)CH3 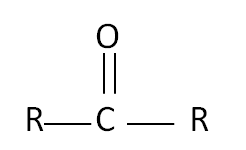 |
Solubility
Carbonyl compounds can form hydrogen bonds with water molecules. The smaller the number of carbons in the compound the more soluble it is in water. Alkyl and aryl groups with many carbons are hydrophobic (water-hating) causing the solubity of the molecule to decrease. An example of a good solvent is propanone.
Shape
The carbon atom has three σ bonds and no lone pairs. The bond angle is 120° causing the shape to be triangular planar.
Boiling point
Carbonyl compounds cannot form intermolecular hydrogen bonds as they do not contain a δ+ hydrogen atom. Because of this, their boiling points are lower than alcohols and carboxylic acids with similar molar masses. Carbonyl compounds are polar which increases their boiling point to higher than that of alkanes and alkenes, with similar molar masses, due to increased dipole-dipole forces. As the number of carbons in the molecule increases, so does the boiling point. The strength of dispersion (intermolecular forces/van der Waals) depends on the number of electrons in the molecule and the amount of contact between molecules.