Aldehydes
Aldehydes are formed by the oxidation of a primary alcohol. The reaction is a distillation. Below is an example of the equipment needed to form ethanal from ethanol.
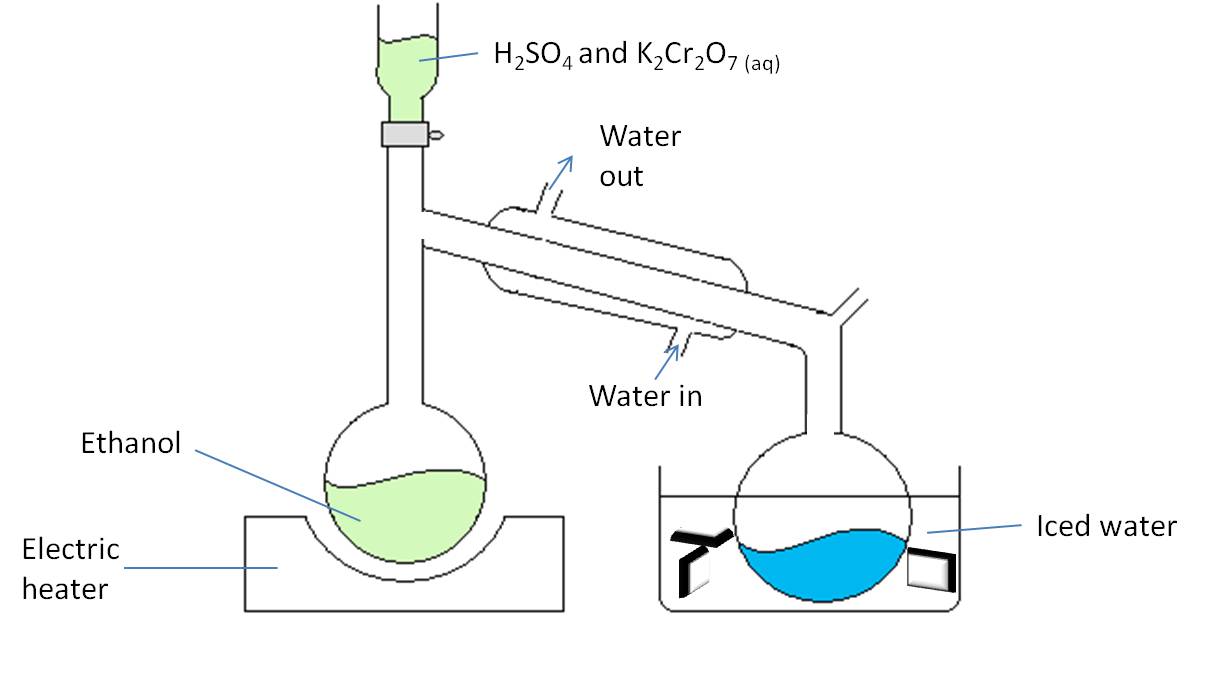
-
Ethanol is placed in a flask and heated to 60°C either using a bunsen burner or an electric heater.
-
The potassium dichromate and sulphuric acid mixture is added in small amounts from the tap funnel.
-
The ethanal is boiled off as it forms and then condenses as it passes down the condenser (which is cooled by water) and it is collected in the receiving flask.
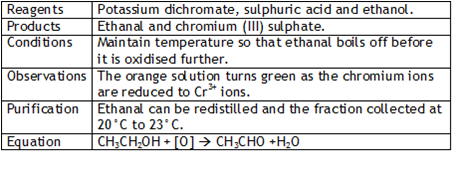
Ketones
Ketones are formed by heating a secondary alcohol under reflux. The equipment below shows the formation of butanone from butan-2-ol.
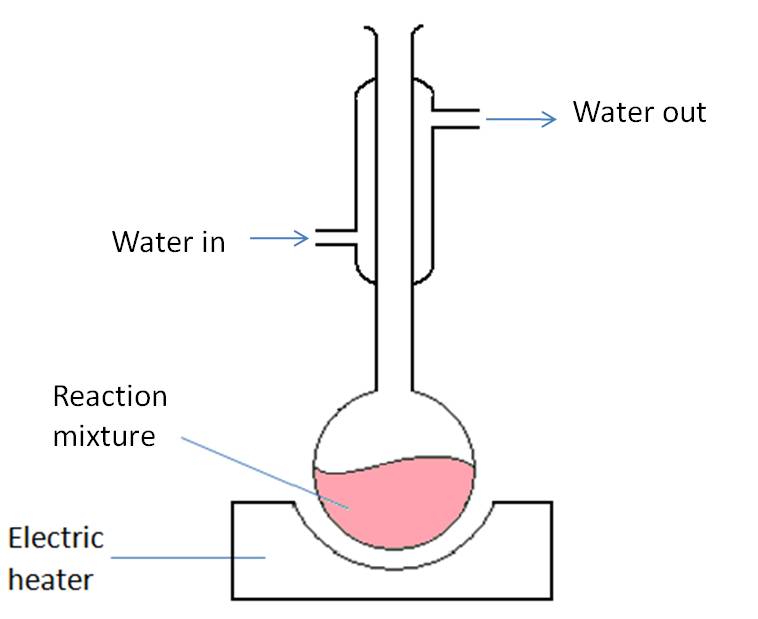
-
Butan-2-ol is placed in a flask with a solution of potassium dichromate and sulphuric acid.
-
A reflux condenser is added on and the mixure is heated. When the mixture starts to boil a stop watch is started and it should continue boiling for 15 minutes.
-
After, the mixture is allowed to cool, then the propanone is distilled off.
