The carbon atom is δ+ causing the C=O bond to be polar. This allows it to be attacked by nucleophiles (a species that donates a lone pair of electrons).
Nucleophilic addition
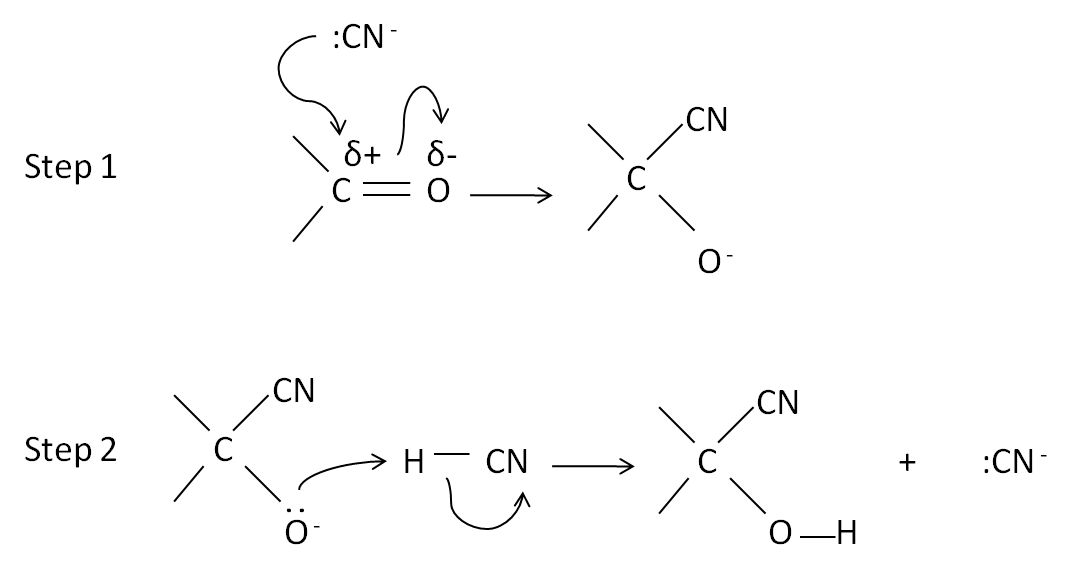
An example of this is the addition of hydrogen cyanide, HCN.
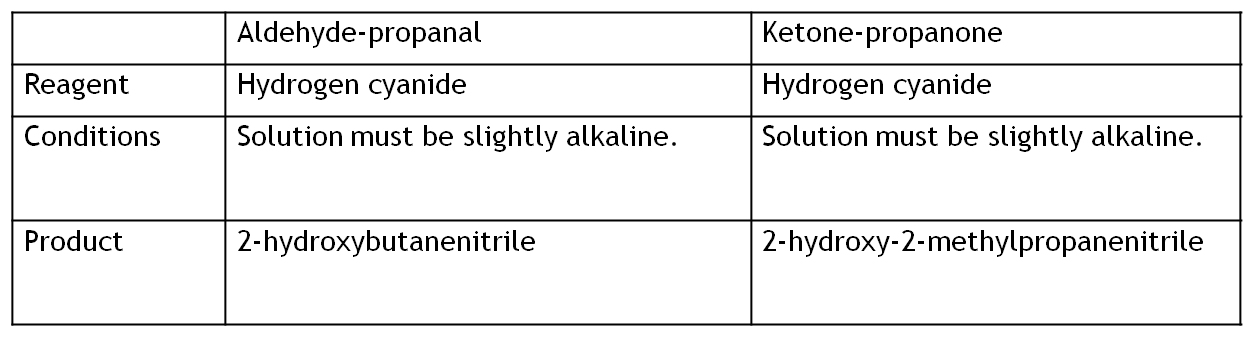
Aldehyde equation
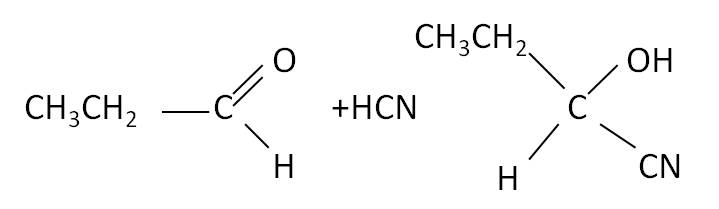 | Ketone equation 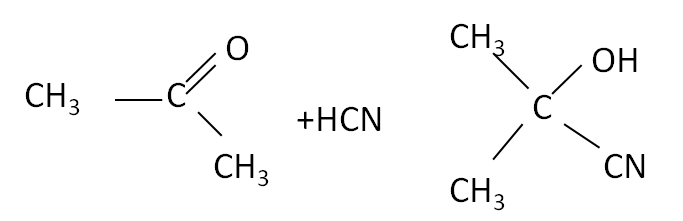 |
Mechanism for addition of HCN
Another example is the addition of hydrogen.
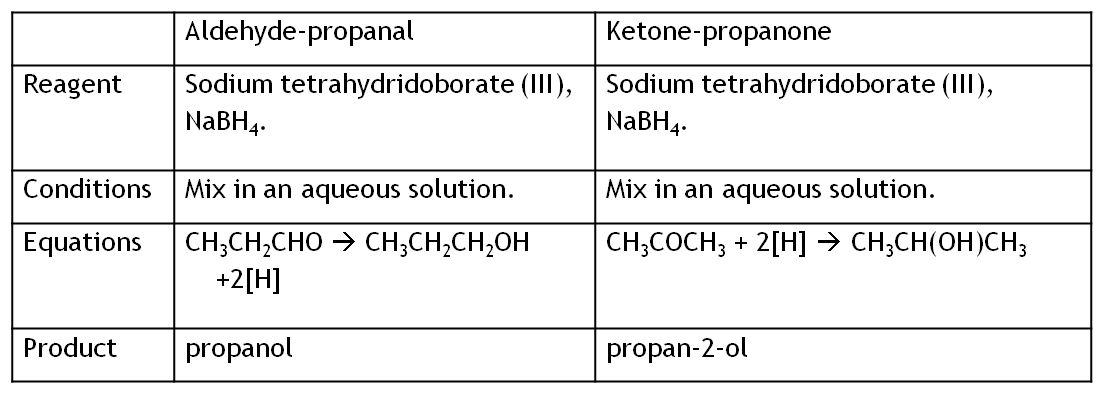
Mechanism for addition of hydrogen
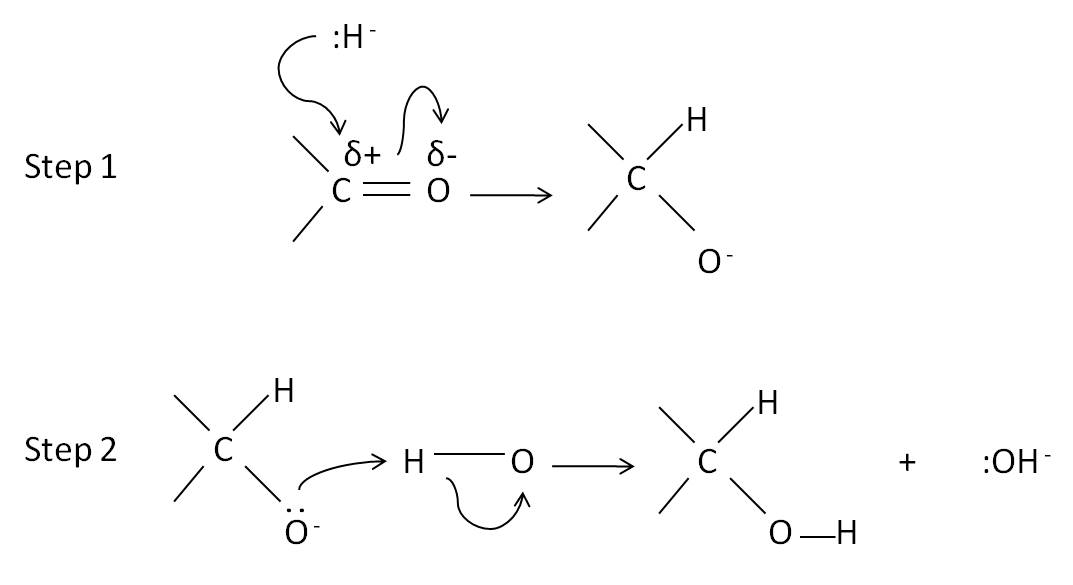
This reaction is a reduction reaction as the carbonyl compound is reduced to an alcohol.
Condensation reaction
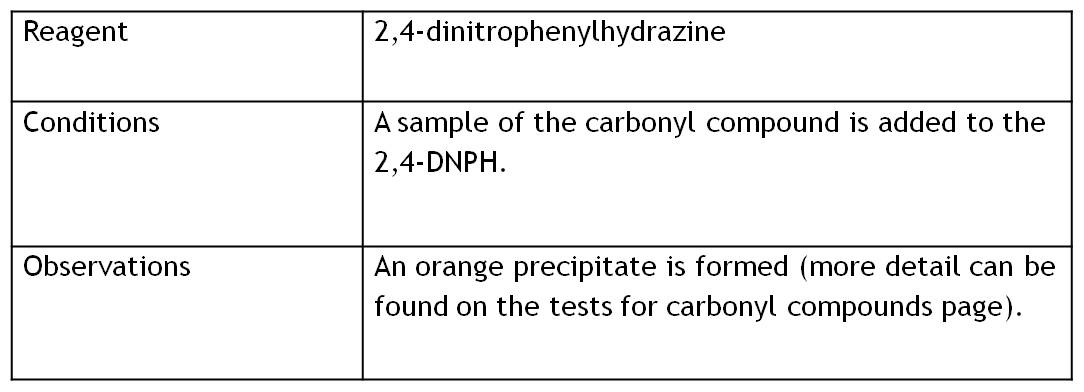
This type of reaction is an addition then an elimination. It occurs in substances with a H2N-NH group. An example of this reaction is using 2,4-DNPH.
Oxidation
Aldehydes can be oxidised to form carboxylic acids.
RCHO + [O] --> RCOOH
-
When using potassium dichromate, the CrO ions (orange) are reduced to Cr ions (green) when mixed with aldehydes but not ketones.
-
When warming carbonyl compounds with Fehling's/Benedict's solution (copper(II) complex), aldehydes form a red precipitate whereas the ketone solution would stay blue.
- When warming carbonyl compounds with Tollens' reagent [Ag(NH3)2]+(aq), a silver mirror is formed with aldehydes as the silver mirror complex is reduced. Ketones cannot reduce this complex.
One last reaction ... The iodoform reaction
A solution of iodine in sodium hydroxide is warmed gently with ketones containing the CH3CO group. A pale yellow precipitate forms (CHI3).